
Description
Byutilizingourinnovativeandproprietarylipidconjugationtechnology,LipoD293(Ver.II)isspeciallydesignedandformulatedbyaddingproprietaryenhancersfortransfectingHEK293cellsandothermammaliancells(Figure1).Asa2ndgenerationliposomebasedDNAtransfectionreagent,LipoD293(Ver.II)offersextremelyhightransfectionefficienciesforHEK293relatedcellsaswellasmanymammaliancellswithlesscytotoxicity.LipoD293reagent(Ver.II),upgradedfromitspreviousversionwithrefinedchemistry,is3~4timesmoreefficientingenedelivery.LipoD293reagent(Ver.II),1.0ml,issufficientfor~666transfectionsin24wellplatesor~333transfectionsin6wellplates.
Figure1.ACartoonShowingLipoD293(Ver.II)EnhancedGeneDelivery
Features
-Topchoiceforhard-to-transfectcells
-Exceptionalhightitersofvirusproduction
-EquallygoodforverylongDNAs(upto180kb)
-EquallygoodforsUSPension293cells(e.g.,293F,293H,etc)
-Highlevelsofrecombinantproteinproduction
-Presenceofserumandantibioticsenhancesefficiencyon293cells
-ExceptionalefficiencyforbothsingleDNAtransfectionandmultiDNAsco-transfection
-Veryaffordable
StorageCondition
Storeat4°C.Ifstoredproperly,theproductisstablefor12monthsorlonger.
ComparisonsofTransfectionEfficiencyofLipoD293DNAInVitroTransfectionReagent(Ver.II)withBrandNameProducts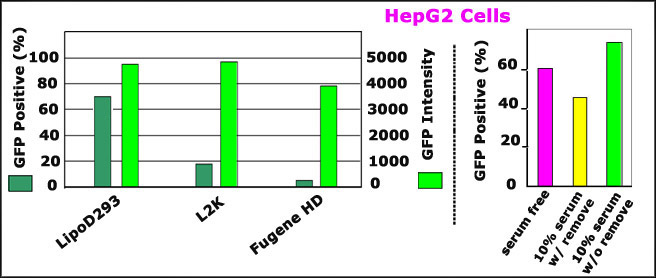
ComparisonoftransfectionefficiencyofLipoD293reagent(Ver.II)vs.lipofectamine2000(L2K)andFugeneHDonHepG2cells.
RightPanel:ComparisonoftransfectionefficiencyofLipoD293(Ver.II)withLipofecatmine2000(L2K),andFugeneHDonHepG2cells.GFPDNA(pEGFP-N3)wastransfectedwithdifferenttransfectionreagentspermanufacturer"sprotocolstoHepG2cell(culturedonCollagenpretreateddishes).GFPpositivecell(%)andfluorescenceintensityweredetectedbypassingthroughFACS48hoursposttransfection
LeftPanel:presenceofserumandantibioticsenhancesLipoD293(Ver.II)efficiencyonHepG2cells.HepG2cell(grownoncollagentreateddishes)wastransfectedwiththreedifferentconditions-------serumandantibioticsfree,presenceof10%serumandantibioticsfollowedbyremoval5hoursposttransfectionandpresenceof10%serumandantibioticswithoutremoval5hoursposttransfection.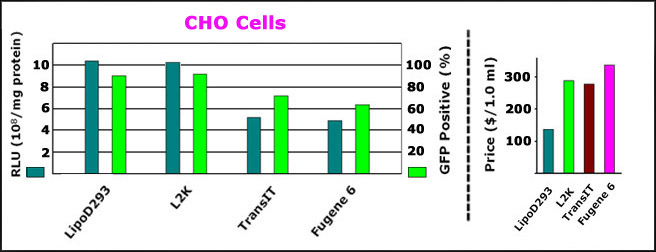
ComparisonoftransfectionefficiencyofLipoD293reagent(Ver.II)vs.lipofectamine2000(L2K),TransITandFugene6onCHOcells.
RightPanel:ComparisonoftransfectionefficiencyofLipoD293(Ver.II)withLipofecatmine2000(L2K),TransITandFugene6onCHOcells.DNAsencodingRenillaluciferase(phRL-CMV)andGFP(pEGFP-N3)weretransfectedwithdifferentDNAtransfectionreagentpermanufacturer"sprotocols.RenillaluciferaseactivityandGFPfluorescenceweredetectedwithRenillaAssaySystemandaNikonEclipsefluorescentmicrocopyrespectively24hoursposttransfection.
LeftPanel:Comparisonofprice($/1.0mlvial)ofLipoD293versusthoseofLipofecatmine2000(L2K),TransITandFugene6.Allthepriceswerecollectedfromthemanufacturers"websites.
AcomparisonoftransfectionefficiencyofLipoD293reagentwithlipofectamine2000(L2K)onahard-to-transfectcell,primaryrataorticsmoothmusclecells.TherataorticsmoothmusclecellswerepreparedandtransfectedwithpEGFP-N3byLipoD293reagent(leftpanel)andLipofecatmine2000(L2K,rightpanel)respectivelypermanufacturers"protocols.ThetransfectionefficiencywasevaluatedbydetectingGFPfluorescencewithaNikonEclipse2000microscopy24hoursposttransfection.TheabovepictureswerekindlyprovidedbyDr.NickolaiDulinofSectionofPulmonaryandCriticalCare,UniversityofChicago.
AcomparisonoftransfectionefficiencyofLipoD293reagentwithFugeneHDonhard-to-transfectcell,LNCapcells.TheLNCapcellsweregrownasATCCrecommendedproceduresandco-transfectedwithpBabe-hygro-SSeCKs(1.5ug)andpEGFP-N3(0.5ug)perwell(6wellplate)LipoD293reagent(leftpanel)andFugeneHD(rightpanel)respectivelypermanufacturers"protocols.ThetransfectionefficiencywasevaluatedbydetectingGFPfluorescencewithaNikonEclipse2000microscopy24hoursposttransfection.TheabovepictureswerekindlyprovidedbyDr.LynGaoofRoswellParkCancerInstitute.
TwoexamplesshowingexceptionalefficiencyofLipoD293reagentonhard-to-transfectcellslikeHepG2andSaoS-2cells.HepG2andSaoS-2cellsin95%confluencyweretransfectedwithpEGFP-N3andpSV-?-galactosidaseDNAsrespectivelyinpresenceofserum/antibiotics.Theefficiencywaschecked48hoursposttransfectionbyZeiss510ConfocalMicroscopyand?-galactosidasestainingkitrespectively.

AcomparisonoftransfectionefficiencyofLipoD293reagentwith293fectinonHEK293cells.HEK293cellstransfectedwithpEGFP-C1plasmidusingLipoD293InVitroDNATransfectionReagent(Ver.II)(upperpanel)andthemostpopularbrandproduct293fectinofInvitrogen(lowerpanel).ThecellswerevisualizedbyNikonEclipseFluorescencemicroscopewithDICphaseimaging(leftpanel)andFITCimaging(rightpanel)24hourspost-transfection.
AcomparisonofLipoD293reagentvs.293fectin,XfectandFugene6transfectionreagentsonproteinproductionwithsuspension293Fcells.30mLof293FcellculturedinstandardculturemediumwastransfectedwithpEGFP-6xHisplasmidusingLipoD293InVitroDNATransfectionReagent(Ver.II)(20ugplasmidDNA),293Fectin(30ugplasmidDNA),Xfect(30ugplasmidDNA)andFugene6(30ugplasmidDNA)permanufacturers"standardtransfectionprotocols. GFPfluorescencewasvisualized48hoursposttransfection(leftpanel)withAforLipoD293,Bfor293fectin,CforXfectandDforFugene6. The6xHistaggedGFPproteinwasthenpurifiedviaNi-NTAaffinitycolumn.5ulof1stelutionfractionwasresolvedonSDS-PAGEfollowedbyCoomassieBrilliantBluestaining(rightupperpanelE)withthelane1forLipoD293,lane2forproteinMarker,lane3forXfect,lane4for293fectinandlane5forFugene6. Theproteinyieldwasquantifiedviaspectrometer(rightlowerpanelF).

AcomparisonofLipoD293(Ver.II)andLipofecatmineLTX(LTX)ongenerationofLentivirus(LV).ThreeCDNAswereco-transfectedwithLipoD293(Ver.II)andLipofectamineLTX(LTX)into293FTcells.AGFPvector,pHR-SIN-cppt-CMVEWP,wasusedtodeterminetiterofLV.1x105293Fcellsperwellwasplatedintoa24wellplatefollowedbyadditionofdifferentofamountsofthevectorsupernatant,1microliter(upperpanel)and10microliters(lowerpanel)respectively.5dayslater,thecellswaspassedwithFACS.Thenumbersattheupperrightcornerindicatethepercentageoftransducedcells.ThetitersofLVgeneratedwithLipoD293andL2Kwerequantifiedtobe8x10^6and3x10^6tu/mlrespectively
TechnicalInformation&Datasheet
-LipoD293(Ver.II)ReagentGeneralProtocol&DataSheet![]()
-LipoD293(Ver.II)ReagentShortProtocol![]()
-LipoD293(Ver.II)ReagentProtocolforTransfectingSuspension293andCHOCells![]()
-LipoD293(Ver.II)ReagentforLentivirusProduction![]()
-LipoD293(Ver.II)ReagentforrAAVProduction![]()
-TechnicalNote&TransfectionTips![]()
Torequestafreetrialsample,pleaseCreateAnAccountwithustoenteryourshippingaddressandemailusatorder@Signagen.com
Testimonials
IamabsolutelythrilledwiththeLipoD293transfectionreagent!IcomparedLipoD293toLipofectaminefortransfectionofplasmidstogenerateviralpseudoparticlesandWOW!Irecovered4timesmorevirususingtheLipoD293reagentthanLipofectamine!!ThisiswonderfulnewsformeandmymentorasitmeansthatIdon"thavetospendsomuchtime(andresources)ontransfecting293Tcellstorecovervirusforinvitroexperiments.MymentorwasalsoveryhappythatLipoD293costsmuchlessthanLipofectamine!!Wehavealreadyordered5mlsofLipoD293andIhavenowconvincedmylabmatestoswitchtoLipoD293sinceIgotsuchgoodresultswithit.Thanksformakingsuchagreatproduct!
--------ChristyLavine,Ph.D.,HarvardUniversity
IwantedtoupdateyouonthefreesampleofLipoD293thatyousenttous.IrecentlytransfectedsomePlat-E"sside-by-sidewithLipofectamine2000,andyourproductout-performedit!!!WearealsovalidatingitwithHeLacells,butotherwiseweareVERYhappywithwhatwehaveseenthusfar!Thankyouforsendingusthefreesample,itdefinatelymadetheSALEforus!
--------CatherineGallo,Ph.D.,UniversityofCincinnati
wehavenowtriedtheLipoD293DNAInVitrotransfectionreagenton293FTcells.InthefirstflaskweusedtheprotocolbyInvitrogenprovidedintheVirapowerboxinsert(Virapower,pBABE-GFPplasmid,plusLipofectamine).InthesecondflaskweusedLipoD293(30ul/6mlmedia)withthesameamountsofVirapowerandplasmidasinthefirstflask.Theresultswerestunning:LipoD293gavetwiceasmanytransformantsasLipofectamine2000...
--------ABetaTesterfromWayneStateUniversity
ebiomall.com






>
>
>
>
>
>
>
>
>
>
>
>
目标蛋白对细胞有毒性,导致细胞死亡;
转染试剂以及DNA用量信息需要优化,否则对细胞具有伤害;
细胞贴壁转染之后没有正常换液。
建议:考虑对目标蛋白进行截短构建、尝试其他细胞系统;摸索转染试剂以及DNA用量信息,如果转染试剂毒性太大,可以考虑尝试义翘转染试剂sinofection;对转染后的细胞进行换液处理,如果细胞状态感觉不够理想,可以考虑添加一些血清来帮助细胞恢复健康。
以上所有分析、建议的前提是,细胞培养、无菌操作等等都没有问题。祝顺利,加油~
不建议你用MDCK细胞,非常难转染。
GFP发出绿色荧光的原理是Ca离子进入GFP的beta-barrel结构中引起的特定能级,因此只要这个结构仍然保持着,就可以发出荧光。
由于GFP的beta-barrel结构非常稳定,一些版本的GFP蛋白(如EGFP)甚至能抵抗94C的高温几分钟而不完全变性,因此想在溶液状态下去掉GFP的荧光是很难的,一般需要用光漂白法。
基于其非常稳定的结构,即便细胞被固定了,仍然会有一部分的GFP蛋白保持其构象而发出荧光。此时荧光可能较弱。在荧光显微镜下是有可能看得到的。
DXY721认为:
悬浮细胞和贴壁细胞在转染过程中差别不大,主要差别在于转染后的筛选,当然如果你做的是瞬时转染就不存在筛选的问题了。
其实转染的过程很简单,问题是能不能转的进去的,转染率能有多少,转进去是否可以稳定表达目的蛋白等等。
我们也是用脂质体做悬浮细胞的转染,说明书上都有具体的操作过程,将脂质体和目的基因按比例混合,然后加到细胞悬液里就OK了,说的简单,实际上还是有一些细节要注意的,比如脂质体和目的基因混合的比例,转染的细胞数,细胞的代数,细胞的状态,有的还要求在转染的前一天传代一次,不过不要怕,这些在脂质体说明书上都有明确的说明,按照说明书做就可以了。
jinghuanlv认为:
悬浮细胞和贴壁细胞转染还是有很大不同的。
脂质体转染的原理基于电荷吸引原理,先形成脂质体-DNA复合物,散布在细胞周围,然后通过细胞的内吞作用,将目的基因导入细胞内,而脂质体复合物与贴壁细胞的接触机会比悬浮细胞高出很多倍,所以,脂质体转染时悬浮细胞的转染效率要明显低于贴壁细胞。
我们实验室转染悬浮细胞是用的电穿孔法,目前为止,悬浮细胞转染的最好方法还是电转,我们实验室用的电转仪是Bio-Rad的,使用条件是电压250V,电容975uF,效果不错,不妨一用。
脂质体是磷脂分散在水中时形成的脂质双分子层,又称为人工生物膜。
阳离子脂质体表面带正电荷,能与核酸的磷酸根通过静电作用将DNA分子包裹入内,形成DNA一脂复合体,也能被表面带负电荷的细胞膜吸附,再通过膜的融合或细胞的内吞作用,偶尔也通过直接渗透作用,DNA传递进入细胞,形成包涵体或进入溶酶体 其中一小部分DNA能从包涵体内释放,并进入细胞质中,再进一步进入核内转录、表达。
转染分2种,一种是瞬时转染,即转染后让细胞表达目的蛋白后即提取蛋白,提一次蛋白,转染一次,这种方式一般不传代;
另一种转染为稳定转染,转染后加入一定选择压力进行筛选,没有转染的细胞不能存活,只留下转染的细胞,这种情况下可以筛选单个转染细胞,构建稳定表达某一特定蛋白或基因的细胞系。









