![absoluteantibody/Anti-F4/80 [Cl:A3-1 (recombinant version)]/200 μg/Ab00106-2.3](images/no_picture.gif)
UniProt Accession Number of Target Protein: Q61549
Alternative Name(s) of Target: EGF-like module; Cell surface glycoprotein F4/80; EGF-like module receptor 1; EMR1 receptor; Em Gpf4; TM7; Ly-71; f480; f-480; f4 80; f 480; f-4/80; f4-80Immunogen: Thioglycollate stimulated peritoneal macrophages of mouse origin.
Specificity: This antibody recognises the mouse F4/80 antigen, a 160kD glycoprotein, expressed by murine macrophages.
Application Notes: The anti-F4/80 antibody, clone Cl:A3-1, has been very widely used in flow cytometry, microscopy and Western blotting since its generation by Austyn and Gordon in 1980. We are the only supplier of recombinant versions of Cl:A3-1 which, whilst having the same variable domains as the original, has been shown to block hybridoma derived anti-F4/80:APC binding to in vitro generated bone marrow-derived (BMDMs) and ex vivo splenic murine macrophages. This was tested using both the recombinant version of the original isotype (rat IgG2b) as well as a murinised version (IgG2a, Ab00106-2.0). Further testing on BMDMs shows that our Fc Silent™ rat IgG2b is a valuable new reagent which shows distinct labelling of F4/80-positive cell populations, whilst the isotype control shows no binding, suggesting that only the antigen binding domains are involved in binding, promoting precision of labelling. The murinised versions will be valuable tools in the study of the role of the murine macrophage in vivo without the artefacts rat-derived antigen being co-administered may harbour. Particularly the murine IgG2a (Ab00106-2.0) should be an excellent candidate for macrophage depletion studies, with the Fc Silent™ (Ab00106-2.3) version being the ideal negative control. Using our recombinant production platform, any species and isotype are possible and our Support Team (support@absoluteantibody.com) will be glad to help you with finding the right variant to match your experimental needs.
Antibody first published in:Austyn JM, Gordon S.F4/80, a monoclonal antibody directed specifically against the mouse macrophage.Eur J Immunol. 1981 Oct;11(10):805-15.PMID:7308288Note on publication:Describes the making of the antibody and its specificity for macrophages (Flow Cytometry) and proposes its potential use as a marker.
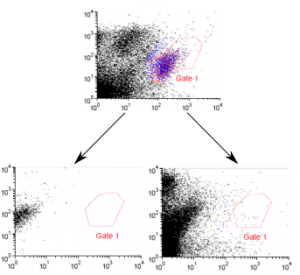
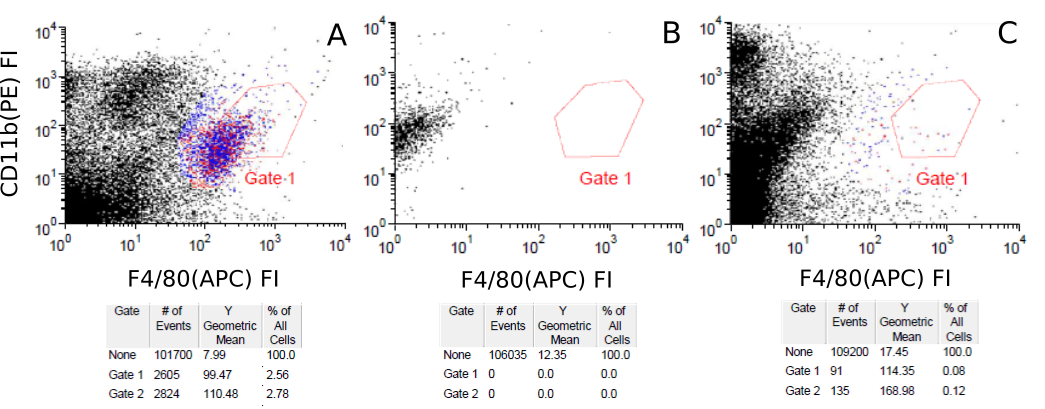
Competitive flow-cytometry assay between Absolute Antibody anti-F4/80 (Ab00106) CI:A3-1 variants and an existing commercial anti-F4/80 antibody. Mouse (Mus musculus) splenocytes were labelled ex vivo with a commercially available APC-labelled anti-F4/80 antibody and APE labelled anti-CD11b antibody and subject to flow-cytometry analysis (A), in which a small subpopulation of F4/80-CD11b positive cells may be observed. Subsets of commerical anti-F4/80 antibody-labelled splenocytes were also subsequently incubated with unlabelled versions of either the rat ( Rattus norvegicus ) IgG2b chimeric version (Ab00106-8.1, B) or mouse IgG2A chimeric (Ab00106-2.0, C) version of CI:A3-1. Loss of the F4/80-CD11b positive subpopulation may be observed, demonstrating displacement of the commercial antibody and the specificity of CI:A3-1.
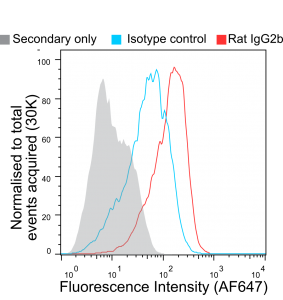
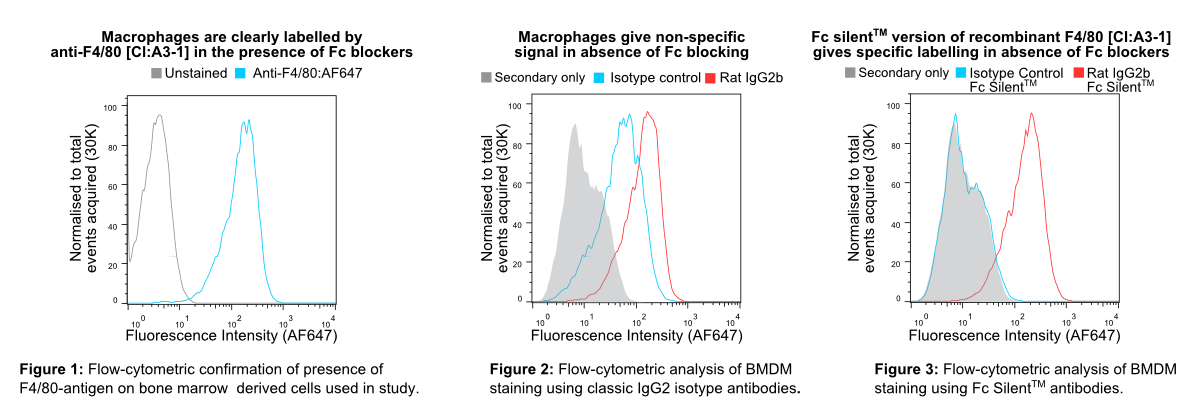
In Figure 1 murine bone marrow-derived macrophages (BMDMs) were pre-blocked with rat anti-mouse CD16 & CD32 (clone FCR-4G8) and stained with non-recombinant anti-F4/80 [Cl:A3-1] conjugated to Alexa Fluor® 647 (AF647), all commercially available from competitors. InFigure 2 BMDMs were stained with recombinant anti-F4/80 [Cl:A3-1] or isotype control (anti-fluorescein [4-4-20 (enhanced)] IgG2b (Ab00102-8.1). InFigure 3 BMDMs were stained with Fc Silent™ recombinant anti-F4/80 [Cl:A3-1] (Ab00106-8.4) or isotype control (Fc Silent™ anti-fluorescein [4-4-20 (enhanced)] IgG2b (Ab00102-8.4). These were fluorescently labelled using the secondary antibody, goat IgG anti-rat IgG (H&L-chain) polyclonal antibody directly conjugated to Alexa Fluor® 647(AF647) commercially available from a competitor. Whilst inFigure 2 the highest fluorescence signal is seen with the recombinant anti-F4/80 IgG2 (the isotype of the original hybridoma-derived antibody), the isotype control IgG2b (Ab00102-8.1) shows considerable signal overlap, indicative of binding of the antibody to Fc-receptors. This illustrates the importance of isotype controls in such experiments when using conventional antibody formats particularly when Fc-blocking reagents are incompatible with the system used due to reactivity with the secondary antibody. The Fc silent™ format however overcomes this issue as seen inFigure 3, where the Fc silent™ recombinant anti-F4/80 (Ab00102-8.4) yields a strong and distinct signal, whilst the isotype control (Ab00102-8.4) shows no discernible difference to the background staining from the secondary antibody alone. Therefore, with Fc Silent™ reagents, no Fc-blocking productsare required. [Data courtesy of Lewis Taylor.]
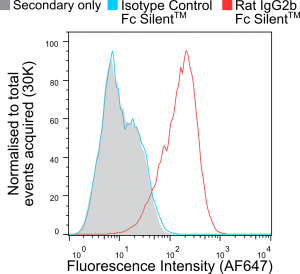
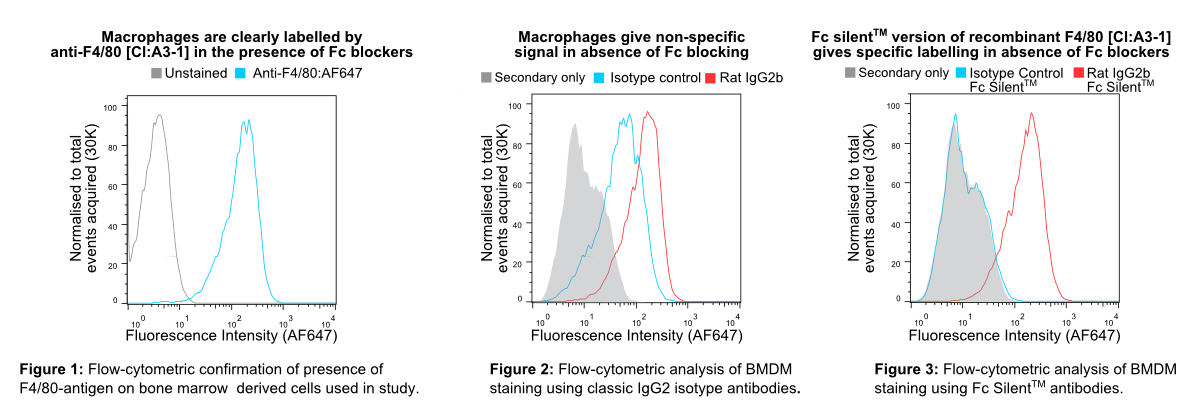
In Figure 1 murine bone marrow-derived macrophages (BMDMs) were pre-blocked with rat anti-mouse CD16 & CD32 (clone FCR-4G8) and stained with non-recombinant anti-F4/80 [Cl:A3-1] conjugated to Alexa Fluor® 647 (AF647), all commercially available from competitors. InFigure 2 BMDMs were stained with recombinant anti-F4/80 [Cl:A3-1] or isotype control (anti-fluorescein [4-4-20 (enhanced)] IgG2b (Ab00102-8.1). InFigure 3 BMDMs were stained with Fc Silent™ recombinant anti-F4/80 [Cl:A3-1] (Ab00106-8.4) or isotype control (Fc Silent™ anti-fluorescein [4-4-20 (enhanced)] IgG2b (Ab00102-8.4). These were fluorescently labelled using the secondary antibody, goat IgG anti-rat IgG (H&L-chain) polyclonal antibody directly conjugated to Alexa Fluor® 647(AF647) commercially available from a competitor. Whilst inFigure 2 the highest fluorescence signal is seen with the recombinant anti-F4/80 IgG2 (the isotype of the original hybridoma-derived antibody), the isotype control IgG2b (Ab00102-8.1) shows considerable signal overlap, indicative of binding of the antibody to Fc-receptors. This illustrates the importance of isotype controls in such experiments when using conventional antibody formats particularly when Fc-blocking reagents are incompatible with the system used due to reactivity with the secondary antibody. The Fc silent™ format however overcomes this issue as seen inFigure 3, where the Fc silent™ recombinant anti-F4/80 (Ab00102-8.4) yields a strong and distinct signal, whilst the isotype control (Ab00102-8.4) shows no discernible difference to the background staining from the secondary antibody alone. Therefore, with Fc Silent™ reagents, no Fc-blocking productsare required. [Data courtesy of Lewis Taylor.]
ebiomall.com






>
>
>
>
>
>
>
>
>
>
>
>
常用流动相加酸碱后PH的总结,希望大家能够提供一点自己测过的结果,谢谢先
1.直接用固体磷酸钠配制成50mM的磷酸钠溶液,再调pH到7.4;(我们试着用这个做了下,发现挂不上柱)
2.配置磷酸钠盐缓冲液:按NaH2PO4:Na2HPO4以19:81的摩尔比配制成pH7.4的缓冲液?(附一张百度出来的配方
)
3.如果是磷酸钠盐缓冲液,可以直接将50mM的NaH2PO4的水溶液用NaOH调成pH7.4吗?
再者,2和3这两个方法配制的磷酸钠盐缓冲液有什么区别?最终效果是一样的吗?如果不一样,有什么理论的知识支撑呢?个人感觉是分析化学中酸碱理论中的缓冲液那里的知识。求帮忙解答这些疑问。
另外,我还想问一下,pH对于Ni柱对His-tagged的蛋白的分离纯化影响大吗?是怎么影响的?谢谢大家了!
有了源数据之后把源数据按照大小排列,
选中源数据区域-->ALT+A1-->选中图标区右键-->更改图表类型-->散点图
因为是考察不同PH对药物的影响,样品又不好改变其PH值,这种情况怎么办?希望有经验的高手指教。
我的流动相是甲醇-水(90:10)
谢谢赐教!
请进子版按格式发贴,自行修改,谢谢。
由弱酸及其盐、弱碱及其盐组成的混合溶液,能在一定程度上抵消、减轻外加强酸或强碱对溶液酸碱度的影响,从而保持溶液的pH值相对稳定。这种溶液称为缓冲溶液。
:)
我在做一细菌不同酸碱度生长状况时,发现这些奇怪现象:pH=3的培养基灭菌(TSB液体培养基)灭菌后pH上升到到9.2!而原来pH=9.0的降到8.7(基本没多少变化),请问各位大侠,这是什么原因?
一般做不同酸碱度生长实验时,该如何才能防止pH在湿热灭菌后基本不变化?
是否可以理解为纯化水得PH范围为6.3-7.6?能否直接用pH计测量?谢谢!









