
Description
Based on our proprietary polymer synthesis technology, PolyJet DNA In Vitro
Transfection Reagent is formulated as a biodegradable polymer based DNA transfection reagent that
ensures effective and reproducible transfection on HEK293,
COS-7,
NIH-3T3, HeLa, CHO and a broad ranges of hard-to-transfect mammalian cells. PolyJet reagent is able to
immobilize DNA migration during electrophoresis at very low concentration and
form transfection complex within 5 minutes at RT. A remarkable feature of the
reagent is the rapid and complete degradation of polymer after transfection
complex endocytosis (Figure 1), leading to much less cytotoxicity. PolyJet
reagent, 1.0 ml, is sufficient for ~667 transfections in 24 well plates or ~333
transfections in 6 well plates, providing a very affordable alternative to the
leading products for transfecting a variety of commonly used and
hard-to-transfect mammalian cells.

Figure
1. A Cartoon Showing Biodegradation of PolyJet
DNA Transfection Reagent After Endocytosis of Transfection Complex
Features
- Bio-degradable
after endocytosis
- Exceptional high titers of virus production
- Equally good for very long DNAs (>89 kb)
- Equally good for both single DNA transfection and multi DNA
co-transfection
- High levels of recombinant protein production
- Simple & robust transfection procedure
- Very affordable
Storage
Condition
Store at 4 °C. If stored properly, the product is stable
for 12 months or longer.
|
Cell Lines |
Efficiency (% GFP) |
Cell Lines |
Efficiency (% GFP) |
| McArdle 7777 Hep3D SHEP 3T3-442A COS-7 CV-1 D 407 DHD Pro.b 3LL B16-F10 BAEC BHK-21 Ca Ski CaCo2 CHO HCS-2/8 HEK-293 HeLa HLMEC H-MVEC Huh-7D12 ATT20 SK-N-SH C2C12 HepG2 |
65-70% |
hESCs |
70% |
Examples Showing Transfection Efficiency of PolyJet DNA In Vitro Transfection
Reagent on Commonly Used Cells

Transfection
efficiency comparison of PolyJet vs. lipofectamine Plus on Chinese Hamster
Ovary (CHO) cells. HA tagged beta-tubulin cDNA was delivered into CHO cells
with PolyJet (left panel) and lipofectamine Plus (right panel) respectively.
FITC conjugated antibody against HA tag was utilized to pick up HA-beta-tubulin
(Green) while a DM1a antibody was used to detect endogenous alpha-tubulin
followed by probing with rhodamine conjugated secondary antibody (Red). The
above picture was provided by Dr. Shang Yin of University of Texas at Houston
Medical School as courtesy
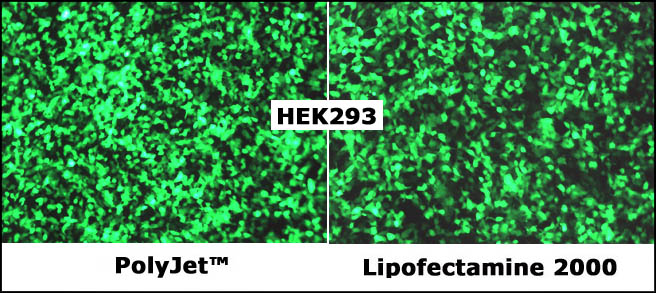
A comparison showing
transfection efficiency of PolyJet reagent vs. a leading product, Lipofectamine
2000 on HEK293FT cells. HEK-293FT cells were transfected with GFP vector
(pEGFP-N3) by PolyJet (left panel) and Lipofectamine 2000 (right panel)
respectively. The cells were visualized by Nikon Eclipse Fluorescence microscope
24 hours post transfection

A comparison showing
transfection efficiency of PolyJet reagent vs. a leading product, Lipofectamine
2000 on HepG2 cells. HepG2 cells were transfected with GFP vector (pEGFP-N3)
by PolyJet (left panel) and Lipofectamine 2000 (right panel) respectively. The
cells were visualized by Nikon Eclipse Fluorescence microscope 24 hours post
transfection

Transfection
efficiency comparison of PolyJet vs. Fugene HD on MDCK cells. A
plain GFP DNA was transduced into MDCK cells with PolyJet (left panel) and Fugene
HD (right panel) reagents respectively per manufacturers' protocols.
GFP and DAPI staining were visualized under fluorescence microscopy 48
hours post tansfection. The above comparison data and pictures were
completed and provided by Dr. Ge Zhou of NYU Medical Center as courtesy

A comparison showing
transfection efficiency of PolyJet reagent vs. a leading product, Lipofectamine
2000 on MDCK cells. MDCK cells are notoriously hard to transfect. With
proprietary "Shaved Cell Transfection" protocol, PolyJet (left panel) gives up
to 70% GFP positive cells vs. Lipofectamine 2000 (right panel) around 5%
efficiency. MDCK cells were transfected with GFP vector (pEGFP-N3) by PolyJet
(left panel) and Lipofectamine 2000 (right panel) respectively. The cells were
visualized by Nikon Eclipse Fluorescence microscope 36 hours post transfection

A comparison showing
transfection efficiency of PolyJet reagent vs. a leading product, Fugene HD on
LNCap cells. LNCap cells were transfected with GFP vector (pEGFP-N3) by
PolyJet (left panel) and Fugene HD (right panel) respectively. The cells were
visualized by Nikon Eclipse Fluorescence microscope 24 hours post transfection

A image showing exceptional
transfection efficiency of PolyJet reagent
on Human embryonic stem cells (hESCs).
The
hESCs grown in E8 medium on Geltrexvs
(left panel, DIC imaging) was transfected with pEF1α-GFP.
The GFP expression (right
panel) was visualized by Nikon Eclipse Fluorescence microscope 24 hours post
transfection.
The above pictures were provided by Dr.
Marina Pryzhkova of
Johns Hopkins University as courtesy

Neuro2A cells transfected
with pEGFP-C1 plasmid using PolyJet In Vitro DNA
Transfection Reagent. The Neuro2A cells were visualized by Nikon
Eclipse Fluorescence microscope with DIC phase imaging (left) and FITC
imaging (right) 24 hours post-transfection
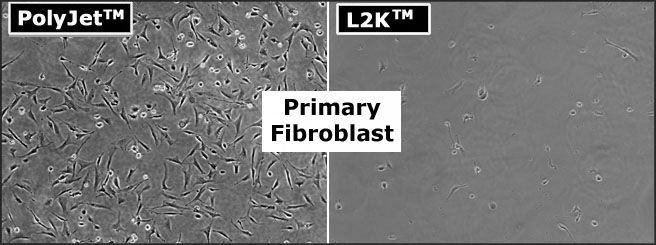
Comparison
of cytotoxicity of PolyJet DNA In Vitro Transfection
Reagent with L2K on primary murine skin fibroblast. The primary murine
fibroblast was incubated with the indicated transfection
reagents/pEGFP-C1 (DNA) complexes above for 4 hours in serum-free DMEM
High Glucose medium followed by replacement of complete
serum-containing medium. The cells were visualized by Nikon Eclipse
Fluorescence microscope with DIC phase imaging 24 hours post
transfection
Data Sheet and Protocols
-
General Protocol for Transfecting Mammalian Cells
![]()
-
A Short Protocol for Transfecting Mammalian Cells
![]()
-
Advanced Protocol for Transfecting
Hard-to-Transfect Mammalian Cells
![]()
-
A Protocol for Transfecting Suspension 293 and CHO
Cells
![]()
-
Protocol for Lentivirus Production
![]()
-
Protocol for rAAV Production
![]()
- Technical Note & Transfection Tips
![]()
Great News! We are offering "Wireless PowerPoint Presenter"
to customers who purchase 5x1.0 ml PloyJet or
more by March 2010. Take advantage of it and don't let this chance go...
|
|
|
FG-3: Wireless PowerPoint Presenter with Case for purchase of 5 X 1.0 ml or more. This presenter is offered for limited time only! |
To request a free trial sample, please
Create An Account with us to enter
your shipping address and email us at
order@signagen.com
![]()
Testimonials:
Sorry
for sooo big delay, but now I am totally in love with PolyJet. I have used
PolyJet for a while on mouse ESCs with relatively good efficiency 50~70%.
--------Marina Pryzhkova, Ph.D., JHU
PolyJet Transfection Reagent worked equally as well as lipofectamine
2000, with little evidence of cell death on 293, PC-3 and 22RV1 cells.
I will defiantly consider switching over.
-----Tiffany Wallace, Ph.D., NCI / NIH
I
only did side by side with the testing sample (PolyJet) and Lipo2000
with GFP transfection on COS-7 cells. The result was very good. PolyJet
was even better than L2K.
------Feng Qiao, Ph.D., NEI / NIH
I tested the sample of PolyJet on my NIH-3T3 mouse fibroblasts this
weekend. The results were much better than Lipofecatmine LTX. I'm
attaching a powerpoint slide with my results (I did not quantify the %
transfection efficiency, but the pictures get the point across). I
found that the protocol for difficult-to-transfect cell lines worked
better than the standard protocol.
-----Stephanie Murphy, Ph.D., Dartmouth College
I had chance to try your product finally. It was great success. I used HeLa
cells and got 10% transfection efficiency (<0.1% for Lipofectamine). Thank you!
I was wondering if I also try GenJet Plus DNA In Vitro Transfection Reagent?
According to your website, the reagent works better than regular PolyJet.
-------Yumi Uetake, Ph.D., UMASS
I tested PolyJet and it looks great on MDCK. We placed order. Thank you!
-------Ge Zhou, Ph.D., NYU
We
are happy to provide feedback. PolyJet worked very well for us in HepG2
cells, we got approximately 80% efficiency with pMAX GFP plasmid, by
following the conditions in your suggested protocol. We ran a
comparison with Lipofectamine, which only showed approximately 20-30%
transfection efficiency. We are planning experiments and will be
ordering more soon.
-------Emily Mcallister, PBRC
ebiomall.com






>
>
>
>
>
>
>
>
>
>
>
>
其次要看下你选择单位的规模如何,做的比较好的,还是上海这边的,你可以看下基尔顿生物,原代细胞培养,动物造模,整体课题外包。
不建议你用MDCK细胞,非常难转染。
GFP发出绿色荧光的原理是Ca离子进入GFP的beta-barrel结构中引起的特定能级,因此只要这个结构仍然保持着,就可以发出荧光。
由于GFP的beta-barrel结构非常稳定,一些版本的GFP蛋白(如EGFP)甚至能抵抗94C的高温几分钟而不完全变性,因此想在溶液状态下去掉GFP的荧光是很难的,一般需要用光漂白法。
基于其非常稳定的结构,即便细胞被固定了,仍然会有一部分的GFP蛋白保持其构象而发出荧光。此时荧光可能较弱。在荧光显微镜下是有可能看得到的。
我刚开始做转染,悬浮细胞,分别做过表达和敲减,看了很多文献,大都没有提及转染后是用转染的这同一批细胞同时做pcr,wb,cck8,凋亡,细胞周期;还是说这次转染只做pcr或wb,再转染一次做cck8或细胞周期。剩下的功能试验均同前,转染一次做一次?我养的是悬浮细胞,转染后做cck8这些功能试验前需要离心换液吗?跪谢解答!
可以用移液器将细胞吸出来并高速离心,沉淀重悬于PBS中洗涤,接着就可以裂解提取蛋白了。可以用超声,酶解等等,裂解后离心收集上清。
其次要看下你选择单位的规模如何,上海这边的,你可以看下基尔顿生物,原代细胞培养,动物造模,整体课题外包。
实验室一直都是用日常型质粒抽提试剂盒,转染细胞没问题。
我觉得只要是注意以下2点就可以了:
1,注意大肠杆菌(Escherichia coli)本身的污染,收集菌体沉淀时防止菌液散落,经常用75%的乙醇擦拭手套。
2,最后洗脱时最好使用无内毒素的水,我们是用注射用水的。
无论是小提还是大提我们都是用的日常型的,并没有刻意用转染级的,因为转染量大,去内毒素的操作太麻烦,损失太大。
转染分2种,一种是瞬时转染,即转染后让细胞表达目的蛋白后即提取蛋白,提一次蛋白,转染一次,这种方式一般不传代;
另一种转染为稳定转染,转染后加入一定选择压力进行筛选,没有转染的细胞不能存活,只留下转染的细胞,这种情况下可以筛选单个转染细胞,构建稳定表达某一特定蛋白或基因的细胞系。










